Serialisation
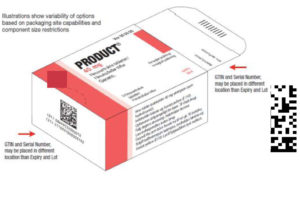
Issues on the introduction of required safety features for medicines
Article 54 of Directive 2001/83/EC of the European Parliament and of the Council of 6 november 2001 on the Community code relating to medicinal products for human use specifies that medicinal products subject to prescription shall bear the safety features that enable the authenticity of medicinal products to be verified and individual packs to be identified.
OGYÉI, in co-operation with HUMVO Plc., the Hungarian Non-profit Company for Medicines Identification (HUMVO Magyarországi Gyógyszer-azonosítási Nonprofit Zrt.-vel), offers up-to-date information on any issues and tasks related to the implementation of these safety requirements.
LETTER OF ANNOUNCEMENT EMVO NOTICE TO FUTURE ON-BOARDING PARTNERS
Joint call of the National Institute of Pharmacy and Nutrition and the Hungarian Medicines Verification Organization Non-profit Plc. (HUMVO) to the Marketing Authorization Holders active on the Hungarian market regarding theregistration/ on-boarding process to the HUMVO
MARKETING AUTHORISATION HOLDER (MAH) REGISTRATION FORM
NMVS Setup Work Packages & Milestones
Leave a reply →

